Lead Sulphate And Sodium Hydroxide Reaction . revision notes on practical: this page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Prepare lead(ii)sulfate for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. Titration must be used if the reactants are soluble. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. soluble salts can be made by reacting acids with soluble or insoluble reactants. which solution(s) could be used to precipitate the barium ion, ba 2+, in a aqueous solution barium nitrate:
from www.numerade.com
revision notes on practical: a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. soluble salts can be made by reacting acids with soluble or insoluble reactants. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. which solution(s) could be used to precipitate the barium ion, ba 2+, in a aqueous solution barium nitrate: Titration must be used if the reactants are soluble. this page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. Prepare lead(ii)sulfate for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams.
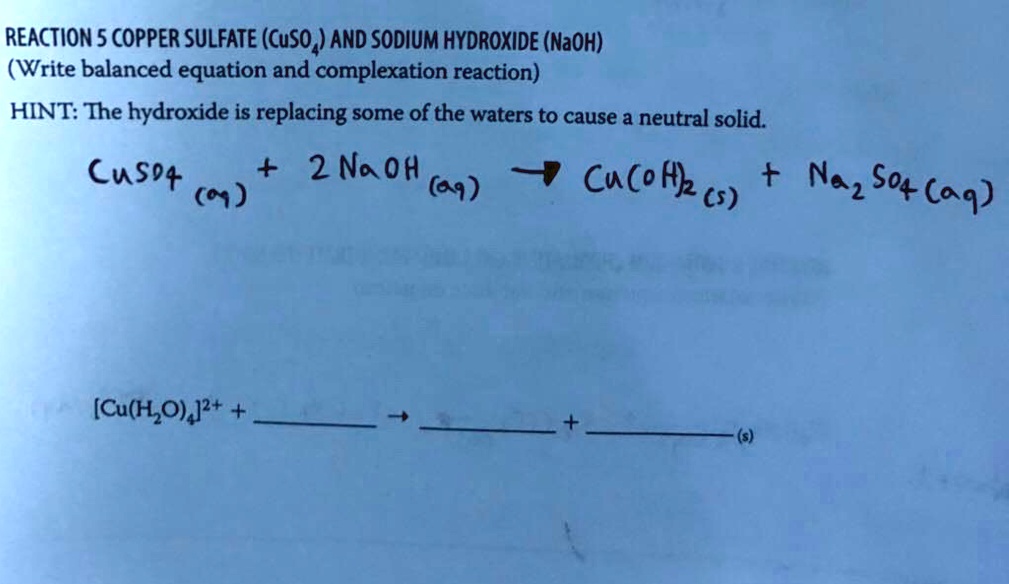
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write
Lead Sulphate And Sodium Hydroxide Reaction Prepare lead(ii)sulfate for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. soluble salts can be made by reacting acids with soluble or insoluble reactants. this page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. Prepare lead(ii)sulfate for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. revision notes on practical: a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Titration must be used if the reactants are soluble. which solution(s) could be used to precipitate the barium ion, ba 2+, in a aqueous solution barium nitrate:
From fity.club
Precipitation Chemistry Chemical Reaction Solution Diagram Lead Sulphate And Sodium Hydroxide Reaction a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. revision notes on practical: this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. soluble salts can be made by reacting acids with soluble or insoluble reactants.. Lead Sulphate And Sodium Hydroxide Reaction.
From www.researchgate.net
Scheme of the sodium sulphate experiment. In the first cycle, the stone Lead Sulphate And Sodium Hydroxide Reaction Titration must be used if the reactants are soluble. revision notes on practical: this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. this page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. which solution(s) could be used to precipitate the. Lead Sulphate And Sodium Hydroxide Reaction.
From dxoezqcmu.blob.core.windows.net
Lead Hydroxide Ionic Formula at Clara Lott blog Lead Sulphate And Sodium Hydroxide Reaction which solution(s) could be used to precipitate the barium ion, ba 2+, in a aqueous solution barium nitrate: this page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. Titration must be used if the reactants are soluble. revision notes on practical: Prepare lead(ii)sulfate for the edexcel igcse chemistry syllabus, written by the. Lead Sulphate And Sodium Hydroxide Reaction.
From www.youtube.com
Reaction of Iron (II) Sulphate (FeSO4) with Sodium Hydroxide (NaOH Lead Sulphate And Sodium Hydroxide Reaction soluble salts can be made by reacting acids with soluble or insoluble reactants. revision notes on practical: a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. this page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. Titration must be used if. Lead Sulphate And Sodium Hydroxide Reaction.
From www.slideserve.com
PPT Reaction of sodium thiosulphate and hydrochloric acid PowerPoint Lead Sulphate And Sodium Hydroxide Reaction Titration must be used if the reactants are soluble. this page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. Prepare lead(ii)sulfate for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. revision notes on practical: soluble salts can be made by reacting acids with soluble or insoluble. Lead Sulphate And Sodium Hydroxide Reaction.
From www.youtube.com
MgSO4+NaOH=Mg(OH)2+Na2SO4. balance the chemical equation Lead Sulphate And Sodium Hydroxide Reaction this page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. Prepare lead(ii)sulfate for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. which solution(s) could be used to precipitate the barium ion, ba 2+, in a aqueous solution barium nitrate: this page looks at the formation of. Lead Sulphate And Sodium Hydroxide Reaction.
From www.numerade.com
SOLVED Ferric chloride is formed by the reaction of iron and chlorine Lead Sulphate And Sodium Hydroxide Reaction a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. soluble salts can be made by reacting acids with soluble or insoluble reactants. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. revision notes on practical:. Lead Sulphate And Sodium Hydroxide Reaction.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube Lead Sulphate And Sodium Hydroxide Reaction revision notes on practical: which solution(s) could be used to precipitate the barium ion, ba 2+, in a aqueous solution barium nitrate: a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead. Lead Sulphate And Sodium Hydroxide Reaction.
From www.meritnation.com
Balanced equation for the reaction between Sodium Hydroxide and Lead Sulphate And Sodium Hydroxide Reaction revision notes on practical: soluble salts can be made by reacting acids with soluble or insoluble reactants. Prepare lead(ii)sulfate for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. which solution(s) could. Lead Sulphate And Sodium Hydroxide Reaction.
From www.dreamstime.com
Double Displacement Reaction Sodium Hydroxide and Copper Sulfate Lead Sulphate And Sodium Hydroxide Reaction soluble salts can be made by reacting acids with soluble or insoluble reactants. a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. which solution(s) could be used to precipitate the barium ion, ba 2+, in a aqueous solution barium nitrate: this page discusses the precipitation of. Lead Sulphate And Sodium Hydroxide Reaction.
From www.numerade.com
SOLVED what happens when sodium hydroxide reacts with sulphuric acid Lead Sulphate And Sodium Hydroxide Reaction which solution(s) could be used to precipitate the barium ion, ba 2+, in a aqueous solution barium nitrate: Prepare lead(ii)sulfate for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. this page discusses. Lead Sulphate And Sodium Hydroxide Reaction.
From www.youtube.com
Equation for PbSO4 + H2O Lead (II) sulfate + Water YouTube Lead Sulphate And Sodium Hydroxide Reaction Titration must be used if the reactants are soluble. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. soluble salts can be made by reacting acids with soluble or insoluble reactants. which solution(s) could be used to precipitate the barium ion, ba 2+, in a aqueous. Lead Sulphate And Sodium Hydroxide Reaction.
From www.slideserve.com
PPT The period 3 elements PowerPoint Presentation, free download ID Lead Sulphate And Sodium Hydroxide Reaction this page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. revision notes on practical: Prepare lead(ii)sulfate for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Titration. Lead Sulphate And Sodium Hydroxide Reaction.
From www.numerade.com
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write Lead Sulphate And Sodium Hydroxide Reaction which solution(s) could be used to precipitate the barium ion, ba 2+, in a aqueous solution barium nitrate: soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants are soluble. a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing. Lead Sulphate And Sodium Hydroxide Reaction.
From www.slideshare.net
Precipitation react2 Lead Sulphate And Sodium Hydroxide Reaction this page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. revision notes on practical: Titration must be used if the reactants are soluble. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Prepare lead(ii)sulfate for the edexcel igcse chemistry syllabus, written. Lead Sulphate And Sodium Hydroxide Reaction.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Lead Sulphate And Sodium Hydroxide Reaction revision notes on practical: Prepare lead(ii)sulfate for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. this page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. soluble salts can be made by reacting acids with soluble or insoluble reactants. this page looks at the formation of. Lead Sulphate And Sodium Hydroxide Reaction.
From www.slideserve.com
PPT Structures of Solids and Liquids PowerPoint Presentation, free Lead Sulphate And Sodium Hydroxide Reaction Titration must be used if the reactants are soluble. a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. revision notes on practical: this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. which solution(s) could be. Lead Sulphate And Sodium Hydroxide Reaction.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Lead Sulphate And Sodium Hydroxide Reaction which solution(s) could be used to precipitate the barium ion, ba 2+, in a aqueous solution barium nitrate: soluble salts can be made by reacting acids with soluble or insoluble reactants. this page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. Titration must be used if the reactants are soluble. a. Lead Sulphate And Sodium Hydroxide Reaction.